Histomorphogenesis of The Cerebellum of The Grey Breasted Helmeted Guinea Fowl (Numida meleagris galeata) Pre and Post Hatch. I 
2 Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
3 Department of Veterinary Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
 Author
Author  Correspondence author
Correspondence author
Animal Molecular Breeding, 2016, Vol. 6, No. 4 doi: 10.5376/amb.2016.06.0004
Received: 24 Mar., 2016 Accepted: 31 Mar., 2016 Published: 31 Mar., 2016
Wanmi N., Onyeanusi B.I., Nzalak J.O. and Aluwong T., 2016, Histomorphogenesis of The Cerebellum of The Grey Breasted Helmeted Guinea Fowl (Numida meleagris galeata) Pre and Post Hatch I, Animal molecular Breeding, 6(4): 1-10 (doi: 10.5376/amb.2016.06.0004)
The cerebellum is a “neuronal machine” that influences primarily motor behaviour, eye movement and conditioning. In this present study, seventy eggs were used for pre and post hatch experiment. At day 11 of the incubation, the cerebellum was observed together with the formation of the ventricular neuroepithelium and the external granular layer (EGL). The cerebellar auricles and vermis appeared by day 18 of incubation and this corresponds to the day the future Purkinje cells was observed. The single row of the Purkinje cells formed between the granular and molecular layer was established at day 26 pre-hatch. Before the 4th and 8th weeks post-hatch, nucleoli, dendrites and distinct cellular components were formed with a strip of EGL persisting.
1 Introduction
The helmeted guinea fowl (Numida meleagris galeata) is a native to Africa and belongs to the Phylum, Chordata; Subphylum, Vertebra; Class, Aves; Order, Galliformes; and Family, Numidae. It is widely distributed in the Guinea Savannah vegetation zone of Nigeria (Ayeni, 1983) and estimated at 44 million in captivity (Ayeni, 1980).
In Nigeria, two types of guinea fowl species are found; Numida ptilorhycha that is indigenous to the Southern part while Numida meleagris is domiciled in the Northern part but is spreading to other small-holder farming areas (Ayorinde, 1987). Some people keep guinea fowl out of curiosity and as “watch animals” around homestead because they have excellent eye-sight, a harsh cry, and they shriek at the slightest provocation (Smith, 2000). They are also kept for income generation (Ligomela, 2000), and for the control of snakes, mice and ticks (Cactus, 2001) thus, encouraging its production. The increase in guinea fowl production has led to the development of informal traders who buy and sale the birds for breeding and consumption, especially during festive seasons (Fajemilehin, 2010).
Birds are subjected to a constant and potentially overwhelming barrage of information from the environment. The development of the brain is an important requirement for the survival of all birds because it controls the entire systems and most importantly in matured birds. It plays a vital function on the skeletal movement properly, through continuous sensory feedback of information concerning the effect of its action (Snell, 2001).
The cerebellum is concerned with sensori-motor effects on the use of limbs (movement), on the eyes.
(sight) and auditory (ear) in relation to the types and morphology of nuclei found within these regions of the brain (midbrain and cerebellum) (Sheibel and Tomiyasu, 1980). The survival of birds, depends on their hearing ability, sight, and precision to correctly identify, process the most important information at every point in time and so as to engage their muscular activity to escape their predators or search for food (Shreesh and Eric, 2011).
Work done on the developmental aspects of avian brain include those by Davey and Tickle (2007) on the brain development of chicken, Salami (2009) skeletal development of guinea fowl, Gossomji (2014) on the development of GIT in guinea fowl and Serdar and Emrah (2010) cortical development of the chicken cerebellum.
However, basic information on the structural development of the cerebellum of the Grey breasted helmeted guinea fowl is not currently available, hence the need for this investigation.
2 Materials and Methods
2.1 Experimental eggs
Seventy (70) fertilized guinea fowl eggs were purchased from National Veterinary Research Institution (NVRI) Vom, Jos, Plateau State, Nigeria. The eggs were transported to a hatchery in Jos and incubated using their standard incubation guide. During incubation, the eggs were turned regularly (minimum of three times) each day for the first 24 days.
Fifty four (54) eggs (two eggs per day) for pre-hatch study were collected daily from day one of incubation up to day twenty eight which is the final day of incubation. An opening was made on the large air space area and the entire egg dropped into a labeled container of 10% buffered formalin for proper fixing. For post-hatch study, brain samples from hatched keets were collected each week for eight weeks and also fixed in 10% buffered formalin.
2.2 Extraction of embryo from egg shell
This was done at pre-hatch using a scalpel blade and clean transparent dish. The blunt side of the scalpel blade was used with the egg held on the palm, and a gentle tap was made on the egg until a crack was formed. Then, the crack was gently widened manually and the embryo collected in a transparent dish.
2.3 Extraction of brain
At pre-hatch, because the entire skull is soft and pliable, scapel blade and rat tooth forceps were used for extraction of the brain. At post-hatch, the keets were euthanized using Nembutal at 40 mg per body weight. Immediately, decapitation was made and the heads fixed in 10% buffered formalin for 3~5 days. After ensuring proper fixation, a dissection was made at the angle of the beak up to the level of the occipital bone. The upper portion of the dissected area is pulled off gradually using the rat tooth forceps until the entire brain is exposed. The cranial nerves were severed to ease the lifting of the brain from the cranium. The extracted brains were fixed in Bouin’s solution comprising 75 mL picric acid, 25 mL formaldehyde and 5 mL glacial acetic acid.
2.4 Separation of the cerebellum
The cerebellum is located on the dorsal portion of the brainstem, with its peduncles: the restiform body connects to the medulla, the brachium pontis connects the cerebellum to the Pons and the brachium conjunctivum connects the cerebellum to the midbrain. The peduncles were severed using a scalpel blade to expose the entire brainstem.
2.5 Gross
Thirty five (27 for pre-hatch and 8 for post-hatch) brain samples were used. Photographs of the dorsal and ventral aspects of the brain were taken using digital handheld Microscope (Magnification 1000x, 5x Zoom, 3D stand high speed DSP).
2.6 Histological techniques
Thirty five (27 for pre-hatch and 8 for post-hatch) brain samples were used. Fixed brain samples were washed using tap water and dehydrated through ascending grades of alcohol (70%, 80%, 90%, 95%, 100%), within the interval of three (3) hours each, cleared in xylene for two hours and embedded in liquid paraffin at 50 °C as described by Kiernan (1990).
Serial transverse sections of 5 µ were made using Jung rotary microtome (Model 42339, Berlin, Germany). Sections were mounted on glass slides and allowed to dry; were deparaffinized, stained, hydrated and cover slipped using diphynylpthalate propylene xylene (DPX) as mountant (Drury and Willington, 1980).
Sections were stained with hematoxylin and eosin (H&E), and cresyl fast violet (CFV) for nuclei and photomicrographs of sections were taken using digital eyepiece (Scopetek DCM 500, Resolution: 15 Pixels, made in China) attached to a light microscope (OLYMPUS XSZ107BN, Hamburg, Germany).
3 Results
3.1 Gross features
The result from this study revealed that during the pre-hatch period, the cerebellum of the grey breasted helmeted guinea fowl (HGF) was observed to develop on the dorsal surface of the pons by the 11th day with the cerebrum, optic lobe, midbrain and medulla oblongata being formed just at the caudal end of the longitudinal fissure, (Figures 1a and 1b). By day 18 of incubation, there was an increased in the size, shape and location of the cerebellum. On this same day, fissures of the vermis first appeared showing various lobes which progressively became distinct. The cerebellar auricles was observed to be attached to the cerebellum at the ventromedial aspects. The rostral part of the cerebellum tapered to fits into the notch that form part of the transverse process (Figures 2a and 2b). The size of the cerebellum tends to increase with increase in size and shape of the cerebral hemispheres at day 27 pre-hatch. Foliation became prominent, rostral part of the cerebellum was broaden and was opposite to what was seen at day 18 pre-hatch. The rostral part became firmly attached to the cerebral hemispheres and optic lobes. Dorsally, the middle cerebellar portion was relatively larger, somewhat spherical to convex but tapered caudally as it joined the pons ventrally. Cerebellar auricles became prominent when viewed from the dorsal and ventral aspects of the brain. The cerebral hemispheres were large and lissencephalic throughout pre and post-hatch but bore some surface structures, such as grooves and eminences, at day 27 (Figures 3a and 3b).
|
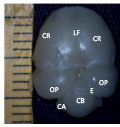
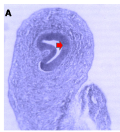
Figure 1a Brain dorsal view showing cerebellum, CH; Cerebral hemisphere, OP; Optic lobe, CB; Cerebellum, M; Medulla oblongata, Magnification, X1000. (Day 11 pre-hatch) |
|
Figure 1b Brain showing ventral view, CR; Cerebrum, MB; Midbrain, OP; Optic lobe, P; Pons, M; Medulla oblongata. Magnification, x1000, (Day 11 pre-hatch) |
|
Figure 2a Brain showing cerebellum, CR; Cerebrum, LF; Longitudinal fissure, E; Transverse fissures, OP; Optic lobe, CB; Cerebellum, CA; Cerebellar auricle (Day 18 of incubation). Magnification X1000 |
|
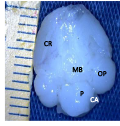
Figure 2b Brain ventral view showing, CR; Cerebrum, MB; midbrain, OP; Optic lobe, P; Pons, CA; Cerebellar auricle (Day 18 of incubation). Magnification X1000 |
|
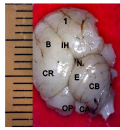
Figure 3a Whole brain indicating; CR; Cerebrum, IH; Interhemispheric fissure, N; Notch, E; Transverse fissures, OP; Optic lobe, CB; Cerebellum, CA; Cerebellar auricle, SP; Spinal cord, (Day 27 of incubation). Magnification X1000 |
|
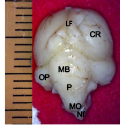
Figure 3b Whole brain indicating; CR; Cerebrum, LF; Longitu dinal fissure, MB; Midbrain, OP; Optic lobe, P; Pons, MO; Medulla oblongata, NF; Nuchal flexure. (Day 27 of incubat Magnification X1000Whole brain indicating; CR; Cerebrum, LF; Longitu dinal fissure, MB; Midbrain, OP; Optic lobe, P; Pons, MO; Medulla oblongata, NF; Nuchal flexure. (Day 27 of incubat Magnification X1000 |
3.2 Histological features
Microanatomical observations on day 11 pre-hatch suggested that the primordium of the cerebellum had been formed and was made up of cerebellar anlage composed of inner and outer mantle layers. Cells within these regions or layers migrated from the ventricular neuroepithelium of the primordium (Figures 4a and 4b). Foliation was observed for the first time on day 13 pre-hatch with granule cells migrating from the external granular layer. The marginal layer was also interposed with migratory cells, (Figure 5). Foliation was completed at this stage and future Purkinje cells were also aligned at the inner cortical layer (Figures 6a and 6b) and (Figure 7).
|
Figure 4a Cerebellum Primordium showing, Arrow: Cerebellar primordium. (Day 11 pre-hatch), Magnification X 140 |
|
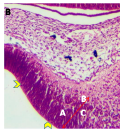
Figure 4b Cerebellar Primordium showing, A; Inner B; Outer mantle, C; Cerebellar anlage, Arrowheads; ventricular neuroepithelium, (Day 11 pre-hatch) |
|
Figure 5 Cerebellum showing, A; Folia formation, B; Marginal layer, C; Inner cortical layer, Arrow; Granule cells migrating from EGL, Day 13 of incubation. H&E. Magnification X140 |
|
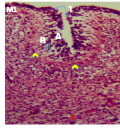
Figure 6a Cerebellum showing, A; External granular layer, B; Primitive molecular layer, 1; Folia deepen, Double arrow; Granule cells migrating from EGL, Red arrow; Large migratory cells, Arrowheads; Future Purkinje cells, Yellow arrow; Folia formed. (Day 18 pre-hatch). H&E. Magnification X140 |
|
Figure 6b Cerebellum showing, A; External granular layer, C; Inner cortical layer, Yellow arrows; Folia formation, Red arrows; Large migratory cells, Dinner granular layer, (day 20 pre-hatch). H&E. Magnification, X140 |
|
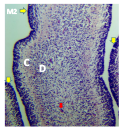
Figure 7 Cerebellum showing, A; External granular layer, B; Dark stained inner granular cells, Red arrow; granule cells migrating from EGL, Arrow heads; Future Purkinje cells, Ox blood arrows; Large granular cells of inner cortical layer. (Day 20 of incubation). H&E. Magnification X140 |
The cerebellum at day 23 was observed to have numerous large immature migratory cells emerging from the dark stained granule cells toward the inner cortical layer. The molecular layer began to appear, numerous neuropils and future Purkinje cells became attached to the neurofibres (Figures 8 and 9). The molecular, granular and Purkinje layers were distinct at day 27 pre-hatch with the presence of immature Purkinje cells arranged in a row and traces of external granular layer still prominent.
|
Figure 8 Cerebellum showing, A; Dense dark stained cell of EGL, B; Molecular layer, C; Granular cell layer, D; White matter, Arrowheads; Larger migratory cells, Arrows; Migratory Purkinje cells, Arrows; Immature Purkinje cells, (Day 23 of incubation). H&E. Magnification X140 |
|
Figure 9 Cerebellum HGF showing, A; EGL, B; Molecular layer, C; Granular layer, Arrowhaeds; Neuropils, Arrows; Migratory Purkinje cells. (Day 24 of incubation). H&E. Magnification X140 |
Cellular organization at the fourth week post-hatch showed that the large perikarya Purkinje cells were round, pear-shaped an arranged in single row at the junction between the molecular and granular layers. The cells demonstrated prominent nucleoli and nuclear materials. Different sizes and shapes of various neurones were observed at 8th week. The inner granular layer was observed to contain numerous small round granule cells giving the appearance of tightly packed chromatic nuclei. The white matter was seen to have sparse granule cells with dense nerve fibres (Figures 10a and 10b) and (Figure 11).
|
Figure 10a Cerebellum of the GF showing, 1; Purkinje cell, 2; Fibre tracts, Yellow arrow; Basket cell, Blue arrow; Nuclei of Purkinje cell, (Week 4 Post-hatch). H&E, Magnification; X400 |
|
Figure 10b Cerebellum of the GF showing, Arrowhead; Stellate cell, Black arrow; Nerve cell, (Week 4 Post-hatch) CFV, Magnification: X400 |
|
Figure 11 Cerebellum of the HGF showing, (A): Arrowheads: Dendrites, 4; White matter, H&E (8 weeks post-hatch) Magnification X 140 (B): 1; basket cell, 2 and 3; Purkinje cells, Arrowheads; Dendrites, H&E (8 weeks post-hatch) Magnification X200 (C): Arrow; Stellate cell, CFV (8 weeks post-hatch) Magnification X200 |
4 Discussion
Cerebellar development is unique because its dysfunction leads to pronounced disturbances in movement, posture and balance since it houses most of the neurones found in the brain. In the grey breasted HGF, the cerebellum was first observed on the dorsal surface of the pons on day 11 of pre-hatch. In the domestic chicken, Serdar and Emrah (2010) observed that the cerebellum was first noticed on the 7th day of incubation. These variations might be as a result of differences in their length of incubation, 21 days for the domestic chicken which will require shorter days to have the cerebellum formed as against the 28 days for the HGF. Cerebellum develops after closure of the neural tube from the fourth vesicle of the five vesicles on the dorso-lateral part of the alar plates of the metencephalon (Mail and Reckess, 2002). On day 18 and 27 of incubation, there occurred as a result of the increase in size, shape and orientation of the cerebellum. These changes might have occurred as a result of the increase in the temporal part of the skull so as to accommodate the brain. The vermis progressively becames distinct including the cerebellar auricles. This is in agreement with observations by Portman and Stingelin (1961) that brain weight and size always increased with increase in body size but varied from one species to another. On the 27th day, the middle part of the cerebellum was relatively larger and somewhat spherical to convex dorsally. This is in agreement with the observation of Ransom and Clarke (1974) that the symmetrical limb movement of reptiles was as a result of their well-developed middle cerebellar portion corresponding to the vermis which is well developed in birds that fly than the flightless. The keets of the grey breasted HGF are precusians, suggesting that they need a well-developed vermis which is responsible for limb control. The large cerebellar auricles observed agrees with finding of Sengluab (1952) that the auricle tends to give a greater degree of body orientation in birds. Whitlock (1952a) had traced degenerating secondary fibers to the auricles following lesion of cranial nerve VIII vestibular nuclei. This suggests that auricle in the grey breasted HGF is associated with auditory functions.
Histological observation at 11th day indicated that the primordium of the cerebellum was found to be made up of cerebellar anlage that was composed of inner and outer mantle layers. Hallonet et al. (1990) in their studies on birds reported that numerous compact cells from the cranial mesencephalic vesicle moved caudally to contribute to this developing anlage. The ventricular neuroepithelium and the external germinal layer appeared on the 11th day as opposed to those observed in the chicken (9th day of incubation) (Serdar and Amrah, 2010). These regions are the most active parts that contribute to cerebellar neurogenesis even at post-hatch (FeiRabaend, 1990).
Foliation was completed on day 13 and future Purkinje cells were observed to align at the inner cortical layer on day 18 of incubation. On the 23th day pre hatch, numerous immature migratory cells moved from the dark stained granule cells toward the inner cortical layer. This suggests that prior to hatching, immature Purkinje cells from the deeper layer, the ventricular neuroepithelium, migrated mainly toward the surface of the granular layer as a result of nuclear cells that coalesced in the cortical and marginal layers during early neurogenesis (Altman and Bayer, 1985a). Redies et al. (2002) observed that cell raphes consisted of migrating dark granule cells between the Purkinje cell clusters at intermediate stage of pre-hatch in chicken. The molecular and granular layers in this study were prominent but the single row of immature Purkinje cells, non-distinctive nucleoli and traces of the EGL were only eminent on day 26 of incubation. This varies with the observation of Espinar et al. (1997) in chicken in which the Purkinje cells formed a single row on day 17 of incubation.
At week 4 post-hatch, Purkinje cells appeared as pear-shaped in a single row at the junction between molecular and granular layers and displayed prominent nucleoli, dendrites and centrally located deeply-stained nucleoli.
5 Conclusion
In the grey breasted HGF, cerebellum was first observed on day 11 of incubation where the cerebellar anlage and the ventricular neuroepithelium were formed. Fissures, vermis, foliation and single row of Purkinje cells were formed during the pre-hatch. The EGL persisted for a short while but thined out a month after hatching.
Acknowledgement
My sincere appreciation to Mr Tobias P. Choji, NVRI Vom; Mr Peter and Mr Bamidele, both of the Faculty of Medicine, A.B.U., Zaria.
Altman J. and Bayer, S., 1985a, Embryonic development of the rat cerebellum. I. delineation of the cerebellar primordium and early cell movements. J. Comp. Neurol. 231: 1-26
http://dx.doi.org/10.1002/cne.902310103
Ayeni J.S.O., 1980, The biology and utilization of the helmeted guinea fowl (Numida meleagris galeata, Pallas), Ph.D Thesis, University of Ibadan, Ibadan, Nigeria
Ayeni J.S.O., 1983, The biology and utilization of helmeted guinea fowl (Numida meleagris galeata pallas) in Nigeria. II, Food of helmeted guinea fowl in kainji Lake basin area of Nigeria, Tropical Agriculture of Ecology, 21 (1): 1-10
http://dx.doi.org/10.1111/j.1365-2028.1983.tb00307.x
Ayorinde K.L., 1987, Characteristics and genetic improvement of the grey breasted helmeted guinea fowl, Numida meleagris galeata pallas in for growth and meat production, Ph.D Thesis, University of Ibadan, Nigeria
Cactus R., 2001, Guinea fowl assortment, Available in:
Clarke P.G.H., 1974, The organization of visual processing in the pigeon cerebellum, Journal of Physiology, 243: 267-285
http://dx.doi.org/10.1113/jphysiol.1974.sp010753
David M.G. and Tickle, C., 2007, The chicken as a model for embryonic development, cytogenic and genome research, 117: 231-239
Drury R.A. and Wellington E.A., 1980, Carletons Histological Technique 5th, Oxford University Press, London, New York Toronto, 1: 653-661
Espinar A., Piera V., Carmona A., and Guerrero J.M., 1997, Histological changes during development of the cerebellum in the chick embryo exposed to a static magnetic field, Bioelectromagnetics, 18: 36-46
http://dx.doi.org/10.1002/(SICI)1521-186X(1997)18:1<36::AID-BEM7>3.0.CO;2-6
Fajemilehin S.O.K., 2010, Morphostructural characteristics of three varieties of grey breasted helmeted guinea fowl in Nigeria, International Journal of Morphology, 28(2): 557-562
http://dx.doi.org/10.4067/S0717-95022010000200036
Feirabend H.K.P., 1990, Development of longitudinal patternsin the cerebellum of the chicken (Gallus Domesticus): A Cytoarchitectural study on the genesis of cerebellar modules, European Journal of Morphology, 28(2-4): 169-223
Gosomji I., 2014, Morphogenesis of the gastrointestinal tract of the helmeted guinea fowl (Numida meleagris galeata), M.Sc Thesis, Ahmadu Bello University, Zaria
Hallonet M.E., Teillet M.A., and LEDourin, N.M., 1990, A new approach to the development of the cerebellum provided by the quil-chick marker system, Development, 108: 19- 31
Kiernan J.A., 1990, Histological and Histochemical Methods: Theory and Practice, Oxford Pergamon Press, London, pp.320-344
Ligomela B., 2000, Population growth compatible with sustainable development, The Zambezi Newsletter, Musokotwane Environment Resource Center for Southern Africa, pp.3
McMaho B. and Bradley B., 1990, The wnt-1 (int-1) proto-oncogene is required for the development of a large region of the mouse brain, Cell, 62: 1073-85
http://dx.doi.org/10.1016/0092-8674(90)90385-R
Mial R.C. and Reckess, G.Z., 2002, The cerebellum and the timing of coordinated eye and hand tracking, Brain and Cognition, 48: 212-226
http://dx.doi.org/10.1006/brcg.2001.1314
Portmann A. and Stingelin W., 1961, The Central Nervous System, In: (Marshall, A.J. (Edition), The Biology and Comparative of Birds, Academic Press, New York and London, pp.37-48
http://dx.doi.org/10.1016/B978-1-4832-3143-3.50006-X
http://dx.doi.org/10.1016/B978-1-4832-3143-3.50007-1
Redies C., Luckner, R., and Arndt, K., 2002, Granule cell raphes in the cerebellar cortex of chicken and mouse, Brain Research Bulletin, 57(3/4): 341-343
http://dx.doi.org/10.1016/S0361-9230(01)00724-9
Salami S.O., 2009, Studies on the Onset of Osteogenesis in Grey Breasted Guinea Fowl (Numida meleagris galeata), P.hD Thesis, Ahmadu Bello University, Zaria, Nigeria
Senglaub K., 1963, Das kleinhirn der vogel in beziehung zu phylogenetischer stellun, lebensweise und korpergrosse, Zwiss Zoological, 169: 1-63
Serdar A. and Emrah S., 2010, The development of chicken cerebellar cortex and the determination of AgNOR activity of the Purkinje cell nuclei, Belg. J. Zool., 140(2): 216-224
Sheibel A.B. and Tomiyasu, U.A., 1980, Dendritic Vascular relationship in the substantia nigra, Experimental Neurology, 70: 717-720
http://dx.doi.org/10.1016/0014-4886(80)90198-3
Shreesh P.M. and Eric I.K., 2011, The role of a Midbrain network in competitive stimulus selection, Elsevier, 21: 653–660
Smith J., 2000, Guinea fowl, Diversification Data Base, Scottish Agricultural College, Available in: http://www.sac.ac.uk/management/external/diversificationtableofcontents, Date accessed: 24th November, 2001, pp.3
Snell R., 2001, Clinical Anatomy for medical students, 7th edition, Washington and Philadelphia, Lippincot Williams and Wilkins Press, pp.701-720
Whitlock G.C., 1952, Sturkie’s Avian Physiology, 5th Edition- Academic Press: University of Hawaii at Manoa, Honololu, USA, pp.704
. PDF(824KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. WanmlWanmi N.
. Onyeanusi B.I.
. Nzalak J.O.
. Aluwong T.
Related articles
. Histomorphogenesis
. Cerebellum
. Helmeted Guinea Fowl
Tools
. Email to a friend
. Post a comment

.png)
.png)
.png)
.png)
.png)
.png)


